Forgot your password?
By Michael McGarvey,
Interventional Fellow & Clinical Research Fellow, King’s College Hospital NHS Foundation Trust
INTRODUCTION
The use of intracoronary (IC) imaging when undertaking percutaneous coronary intervention (PCI) is increasingly recognised as ‘standard of care’. International guidelines state, with Class IA recommendation, that IC imaging should be used in all patients with high anatomical complexity (e.g., left-main stem, long lesions, and bifurcations) across the clinical spectrum of coronary artery disease (CAD) (1, 2). IC imaging use reduces target lesion failure (TLF) by almost one third and all-cause mortality by one quarter compared to interventions guided by angiography alone (3). United Kingdom interventional cardiologists have responded to this impressive data accordingly – in patients with complex anatomical lesions, the use of IC imaging doubled between 2014 and 2020, and this trend will surely continue (4).
However, realising the benefits observed in randomised controlled trials (RCTs) in real-world practice may be challenging. IC imaging must be applied and interpreted in a systematic manner, akin to the highly protocolised studies that have demonstrated such dramatic benefits.
In this editorial, we will review the key RCTs that have demonstrated the superiority of IC imaging over angiographic guidance; we will explore the validated criteria for PCI optimisation and their importance; and finally, we will discuss the role that our society can play as we work to translate the observed benefits from RCTs into routine clinical practice.
Current GUIDELINES & Evidence For Use
Intracoronary imaging, with intravascular ultrasound (IVUS), was first introduced to clinical practice in 1989 due to the recognition that coronary angiography lacks the requisite spatial resolution to guide and optimise PCI (5). Intracoronary optical coherence tomography (OCT) was first reported in 2002 (6). IVUS, an ultrasound-based modality, provides superior depth of penetration (~10-12mm) at the expense of reduced axial resolution (100-200µm.) OCT, which uses infra-red light in the electromagnetic spectrum, provides improved spatial resolution (at 5-20µm), but reduced depth of penetration. Additionally, image acquisition requires clearance of blood from the lumen (achieved through injection of iodinated contrast), limiting its application in large diameter vessels and aorto-ostial lesions (7).
Since 2015, almost 20,000 patients have been included in international randomised controlled trials (RCTs) comparing IC imaging against angiographic guided PCI. These studies have consistently demonstrated the superiority of IC imaging (Table 1) (8-15), with reductions in target lesion revascularisation (TLR), myocardial infarction (MI), and all-cause death when combined in meta-analysis (3). [INSERT TABLE 1]
Recruitment to these trials focussed on those patients and lesions with features associated with an increased risk of stent failure, including acute coronary syndrome (ACS) culprit lesions, anatomically complex lesions, and patients with increased clinical risk, such as those being treated for type 2 diabetes (13). The ULTIMATE trial was an exception, as it was designed as a study of ‘all-comer’ patients requiring drug eluting stent (DES) implantation (10). However, it should be noted that despite this broader inclusion criteria, the mean lesion length was ~35mm and two-thirds of patients had lesions that were classed as ‘complex’ according to the AHA/ACC classification of lesion morphology (16, 17).
Clinical outcomes do not appear to differ significantly according to the choice of IC imaging modality, despite the inherent differences in the underlying technologies. When IVUS and OCT have been compared directly in RCT, or indirectly through network meta-analysis, outcomes are comparable (18, 19). Selection of IC imaging modality may therefore be determined by available resources, operator experience, and clinical scenario. International guidelines state that IVUS may be preferred in the assessment of the left-main coronary artery (1), whilst OCT may be preferred in the assessment of patients with myocardial infarction and non-obstructed coronary arteries (20).
IC IMAGING guidance or optimisation?
The evidence supporting the routine adoption of IC imaging when undertaking PCI in anatomically complex lesions is now clear, and international guidelines have been updated accordingly. What is less clear, is whether these impressive benefits can be realised in routine clinical practice, where operator confidence in image interpretation remains a concern and resources, especially time, are limited (21).
Central to the success of each of these RCTs was a strictly protocolised approach to the application of IC imaging, to satisfy two key goals: (i) to maximise stent absolute and relative stent expansion, with guidance on device sizing, stent optimisation, and lesion coverage; and (ii) appropriate correction of early post-PCI complications, including edge dissection. Operators were provided with an itemised checklist that they were required to satisfy to achieve an IC imaging optimised PCI.
In the ULTIMATE study, comparing IVUS vs. Angiographic guided PCI in ‘all-comers’, an ‘optimal’ result required: a) a minimal stent area (MSA) of >5.0mm2 or 90% of the distal reference; b) plaque burden less than 50% in the 5mm proximal and distal to the stent edge; and c) no edge dissection involving media with length >3mm (7). In OCCUPI, where OCT was compared against angiography, similar definitions were applied, with optimisation criteria defined as: a) MSA ≥80% of the mean references lumen areas, or MSA ≥100% of distal reference, or MSA >4.5mm2; b) stent strut malapposition <400µm; and c) absence of a major edge dissection (i.e., circumference of dissection >60°, dissection length <3mm, and absence of deep vessel injury (15). The protocols for RENOVATE-COMPLEX (11), IVUS-ACS (14), and OCTOBER (12) applied similar criteria.
What was consistent across the trials was the apparent importance of achieving the criteria for optimisation. In ULTIMATE, the incidence of the primary endpoint was just 1.6% at 1 year when all three criteria were satisfied, as opposed to 4.4% when this was not the case (or 5.2% when angiographic guidance was used.) In OCCUPI, if optimal OCT guided stent placement occurred, rates of 1-year TVF were just 2.9%, compared to 8.6% with sub-optimal OCT guided stent placement (and 7.4% with angiographic guidance)(15) (Figure 1).
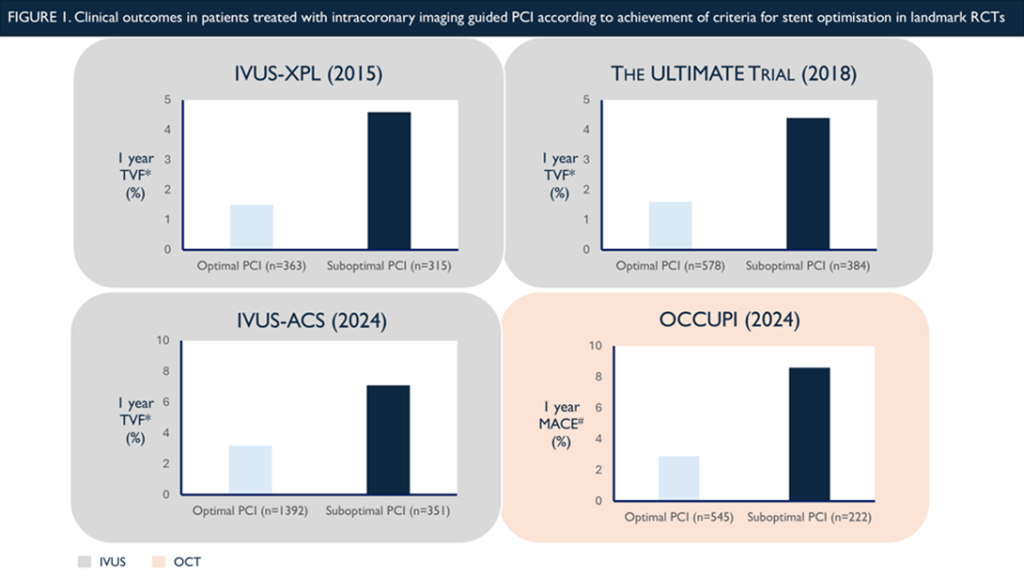
* Target vessel failure (TVF), defined as a composite of cardiac death, target vessel myocardial infarction, or clinically driven target vessel revascularisation
# Major adverse cardiovascular events (MACE), defined as a composite of cardiac death, myocardial infarction, stent thrombosis, or ischaemia-driven target-vessel revascularisation
============================================================================================
Inability to achieve these targets, and the apparent associated adverse outcomes, may simply reflect the complexity and extent of the underlying CAD. An important challenge to this explanation, however, arises from a post-hoc analysis of the OCTOBER study. OCTOBER randomised 1200 patients to bifurcation PCI with OCT or angiography guidance alone. When OCT images were assessed by a core-lab, almost 10% of cases demonstrated evidence of unintended stent deformation (USD). Only half of these were detected by expert operators on-site. When USD was recognised and corrected, there were no associated adverse outcomes at 2 years. Where USD was not recognised and went untreated, 23% of patients (n=30) experienced an adverse outcome. These events accounted for over half of the total events documented in the OCT guided arm (12, 22). While the OCTOBER study was unique in that it only recruited patients undergoing bifurcation PCI, a generalisable lesson is that benefit offered by IC-imaging over angiography in complex anatomical lesions arises from iterative physician interaction with the IC imaging, to identify and correct a sub-optimal result. Failure to do so, may lead to outcomes no better than what can be achieved with angiographic guidance.
IN SEARCH OF ‘OPTIMAL’
This editorial does not intend to provide a glib analysis of the failure of expert operators to achieve an ‘optimal’ PCI result in landmark RCTs. Instead, it aims to highlight the challenge that we all face if we wish to realise the potential benefits offered by IC imaging. In the current era of PCI, a technology that potentially offers a 25% reduction in all-cause mortality compared to standard practices must be embraced, and national societies, such as ours, must play a central role in ensuring widespread adoption.
A recent international expert consensus ‘white paper’ addressing the low utilisation of IC imaging in PCI called for improved educational programmes for trainees and consultant operators, structured reporting of intracoronary imaging guided procedures, and mandatory collection of key post-PCI metrics (21). We are entering an era in which angiographic guided PCI, particularly in anatomically complex lesions, should be seen as an exception. BCIS is perfectly placed to guide our community through this transition, providing the education, guidance and mandated standards to ensure an IC imaging optimised PCI result may be delivered to every .
REFERENCES
TABLE 1. Key Trials Comparing PCI Guided by Angiography Alone vs. Intracoronary Imaging Optimised PCI
| Trial | Enrolled, n
(Randomisation ratio) |
IC Imaging Modality | Follow-up, months | Lesion Complexity | Endpoint | Results | Notes |
| CTO-IVUS (2015) (8) | 402
(1:1) |
IVUS vs. Angio | 12 |
· Chronic total occlusion |
Composite of cardiac death, TL-MI or ischaemia-driven TLR |
5 (2.6%) vs. 14 (7.1%)
[HR 0.35 (95%CI 0.13-0.97) (p=0.035] |
· Primary outcome was Cardiac death; 0 in IVUS vs 2 in Angio only
· Korea only
|
| IVUS-XPL (2015) (9) | 1400
(1:1) |
IVUS vs. Angio | 12 |
· Long lesions ≥28mm |
Composite of cardiac death, TL-MI or ischaemia-driven TLR
|
19 (2.9%) vs. 39 (5.8%)
[HR 0.48 (95%CI 0.28-0.83) (p=0.007)]
|
· 94.5 % follow up @12mnths
· Korea only |
| ULTIMATE (2018) (10) | 1448
(1:1) |
IVUS vs. Angio | 12 |
· ‘All comers’ – 8.9% CTO – 12.6% unprotected Left Main – 34.2% any bifurcation – Avg. stent length ~ 48mm – 24.8% moderate to severe Ca2+
|
Composite of cardiac death, TV-MI or clinically indicated TVR |
21 (2.9%) vs. 39 (5.4%)
[HR 0.53 (95%CI 0.31-0.90) (p=0.02)] |
· Operators minimum 200 PCI/yr
· China only |
| RENOVATE-COMPLEX-PCI (2023) (11) | 1639
(2:1) |
OCT or IVUS vs. Angio | 24 |
· ‘Complex’ lesions – 19.5% CTO – 11.7% unprotected Left Main – 21.9% ‘True’ bifurcation – 54.8% long lesions (≥38mm) – 37.9 % Multivessel PCI – 14.1% severe Ca2+
|
Composite of cardiac death, TV-MI or clinically indicated TVR | 76 (7.7%) vs. 60 (12.3%)
[HR 0.64 (95%CI 0.45-0.81) (p=0.008)] |
· Korea only |
| OCTOBER (2023) (12) | 1201
(1:1) |
OCT vs. Angio | 24 |
· ‘True’ bifurcations with SB diameter ≥2.5mm – 18.9% LMS – 70.5% LAD/D1
|
Composite of cardiac death, TL-MI or ischaemia-driven TLR |
59 (10.1%) vs. 83 (14.1%)
[HR 0.70 (95%CI 0.50-0.98) (p=0.035)]
|
· Europe only
· 15.3% IVUS use in ‘angiography’ arm |
| ILUMIEN-IV (2023) (13) |
2487
(1:1)
|
OCT vs. Angio | 24 |
· ‘Complex’ lesions and/or T2DM – 19.5% CTO – 11.7% unprotected Left Main – 21.9% ‘True’ bifurcation – 54.8% long lesions (≥38mm) – 37.9 % Multivessel PCI – 14.1% severe Ca2+
|
Composite of cardiac death, TV-MI or ischaemia-driven TVR |
88 (7.4%) vs. 99 (8.2%)
[HR 0.90 (95%CI 0.67-1.19) (p=0.45)] |
· 18 countries including Europe, N.America, Asia & Oceania
· Co-primary endpoint of final MSA was 5.72±2.04 vs. 5.36±1.87 (p<0.0001)
|
| IVUS-ACS (2024) (14) | 3505
(1:1)
|
IVUS vs. Angio | 12 |
· Acute coronary syndromes – 27.6% STEMI – 4.3% unprotected Left Main – 15.2% ‘True’ bifurcations – 72.5% long lesions (≥30mm) – 7.6% moderate to severe Ca2+ |
Composite of cardiac death, TV-MI or clinically indicated TVR | 70 (4.0%) vs. 128 (7.3%)
[HR 0.55 (95%CI 0.41-0.74) (p=0.0001)] |
· China (52 centres), Pakistan (2), UK (1), Italy (1)
· Minimum 1000 PCI per site* & 200 PCI per operator
· Patients underwent second randomisation to DAPT vs. Ticagrelor monotherapy
|
|
OCCUPI (2024) (15)
|
1604
(1:1)
|
OCT vs. Angio |
· ‘Complex’ lesions – 7.2% CTO – 20.4% Acute myocardial infarction – 14.3% unprotected Left Main – 23.8% ‘True’ bifurcations – 71.8% long lesions (≥28mm) – 9.3% severe Ca2+ – 8.1% intracoronary thrombus – 10.7% in-stent restenosis
|
Composite of cardiac death, MI, stent thrombosis, or ischaemia driven TLR | 37 (4.6%) vs. 59 (7.4%)
[HR 0.62 (95% CI 0.41-0.93) (p=0.023)] |
· Korea only
· Initial 2×2 factorial analysis with assessment of 3mnth DAPT vs. 12mnth DAPT abandoned due to concern of lack of equipoise in this cohort
|
|
|
Ca2+: Calcification; CTO: Chronic total occlusion; IVUS: Intravascular ultrasound; OCT: Optical coherence tomography; PCI: Percutaneous coronary intervention; STEMI: ST-elevation myocardial infarction; TL-MI: Target lesion myocardial infarction; TLR: Target lesion revascularisation; TV-MI: Target vessel myocardial infarction; TVR: Target vessel revascularisation |
|||||||